
One year ago, LunaVax began with a simple question:
How can we make vaccines easier to deliver, receive, and more reliable where access is hardest?
Today, we are laying the foundation for a new vaccine access system in Nigeria that brings together microneedle vaccine patches (MAPs), last-mile delivery models, and digital and biometric tools to strengthen immunization systems.
This past year has been about building the foundation for a scalable model.
From Question to Foundation
Microneedle vaccine patches hold enormous promise. They can simplify administration, reduce dependence on trained injectors, and improve the vaccination experience for caregivers and children.
But technology alone does not solve access.
Vaccines only save lives when they reach the right person, at the right time, with the right information and follow-up. This is why LunaVax moved beyond building a product to building an integrated access system that connects delivery technologies, operational workflows, and data infrastructure.
Why Nigeria
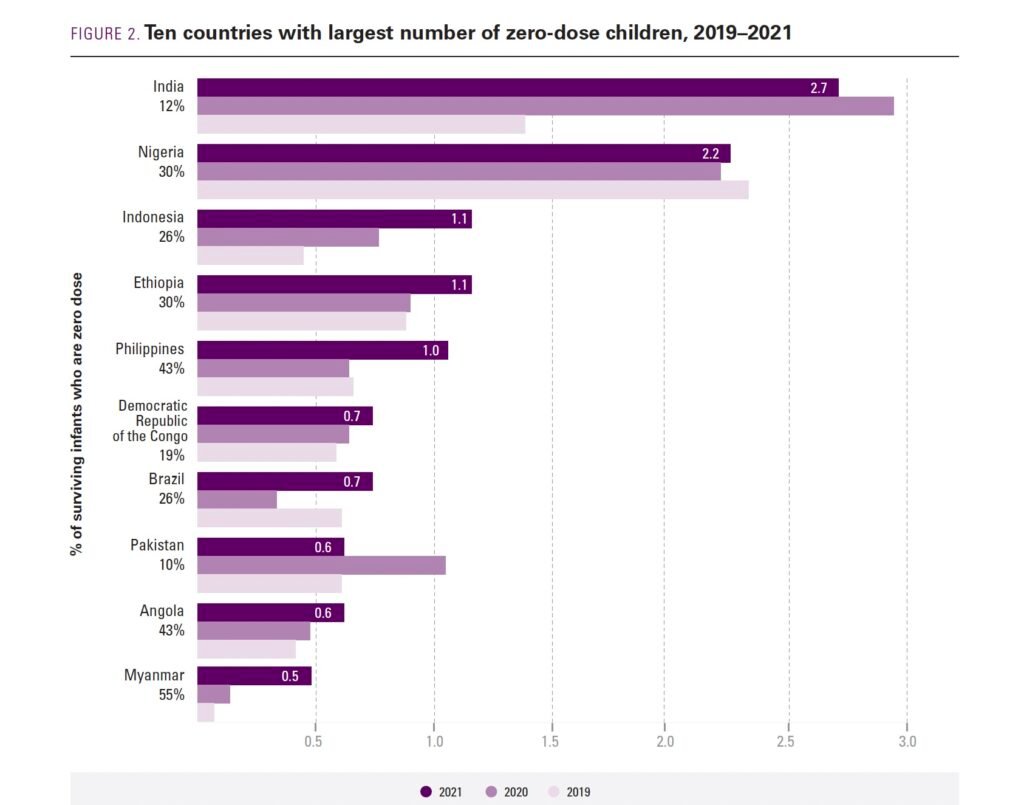
Source: WHO/UNICEF
Nigeria carries one of the largest burdens of zero-dose and under-immunized children globally, while also operating one of Africa’s most extensive public health delivery networks.
If a new access model can function within Nigeria’s scale and complexity, it can work in many other settings.
Over the past year, LunaVax has engaged partners across Lagos, Abuja, and beyond to understand operational realities and shape what feasible implementation could look like in practice.
Nigeria is not just our first site. It is where this model is being proven.
WHO and UNICEF estimate that Nigeria has one of the world’s largest populations of zero-dose children.
Read more →
A Global Conversation: UNGA80

Source: LunaVax
In September 2025, LunaVax participated in global health discussions at the 80th United Nations General Assembly in New York, including The Tipping Point: A New Era for Global Health Leadership, a high-level convening organized by Global Health Corps alongside partners such as Africa CDC, PATH, and The Access Challenge.
The conversations highlighted a clear priority: stronger domestic financing and innovative models for local and regional vaccine production. For LunaVax, UNGA80 marked an important shift, moving the work from concept to action.
From Vision to Evidence
In October 2025, LunaVax officially launched preparatory work for a MAP focused pilot through the Harvard Medical School Global Clinical Scholars Research Training (GCSRT) program, centered on feasibility, usability, and implementation design.
This work explores how MAPs can be used beyond traditional clinic settings, how caregivers and frontline workers experience the technology, and how digital and biometric tools can support continuity and accountability. Alongside this, the Harvard GCSRT capstone is informing early Phase 3 protocol and informed consent concepts and guiding LunaVax’s Nigeria MAP Vaccine Roadmap toward scalable implementation and local manufacturing.
Together, these efforts signal a long-term, evidence-driven commitment to building a scalable and locally grounded vaccine access model in Nigeria.
Next Phase
LunaVax is preparing for a phased MAP and biometrics pilot, focused on site mapping, workflow design, partner engagement, and feasibility and usability studies.
Over the coming year, we will finalize pilot design, strengthen partnerships, and prepare for initial field activities. Our priority is building a model that is implementation-ready and scalable. One year in, a strong foundation is in place.
Interested in partnering or learning more about LunaVax’s MAP and biometrics work in Nigeria?
partnerships@lunavax.com | www.lunavax.com
#BeLunacious

Really great article. I enjoyed reading this and look forward to following LunaVax’s journey.